Taking a step towards continuous
mRNA manufacturing
Benefits
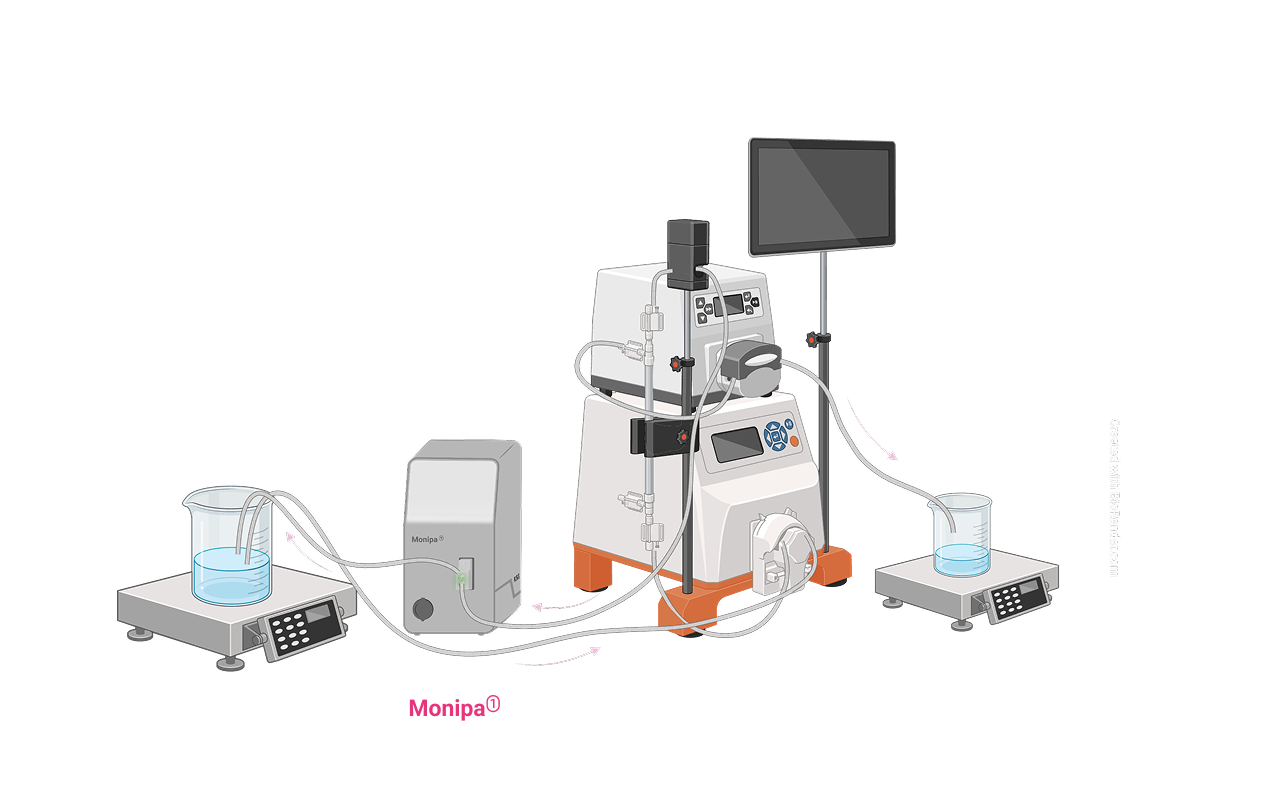
mRNA Manufacturing Process Overview
Related Assets
Real-time, inline quantification of DNA, RNA, and excipients in under a minute with precision. Obtain instant process data, make faster decisions, and boost yields in nucleic acid based therapeutics. Contact us to see how Monipa can be integrated into your operations!
