Overcoming Challenges in mAb
Process Development
Why inline downstream monitoring matters?
Integration of Monipa into mAb Downstream Processing
Inline UF/DF monitoring with Monipa

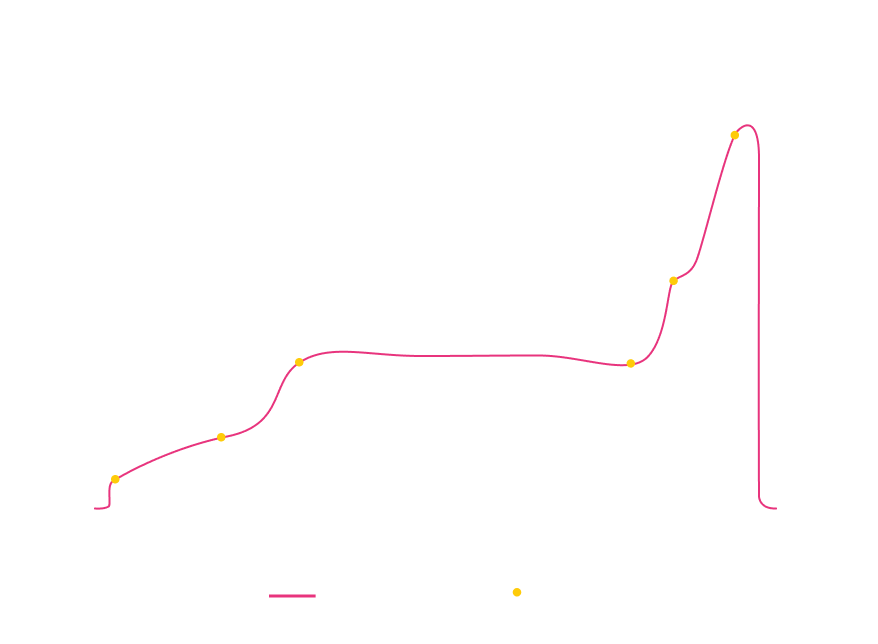
UF/DF is one of the most effective separation techniques and a critical step in downstream processing for precisely achieving the target product composition. The process relies on a permeable membrane to selectively separate components as a specific volume of formulation buffer is exchanged. It can be divided into key stages:
Ultrafiltration 1
Accurate timing of UF1 improves consistency and reduces process variability.
Diafiltration
Moving beyond fixed DF volumes with real-time monitoring optimizes buffer usage.
Ultrafiltration 2
Preventing deviations at this stage helps avoid reprocessing or, in the worst case, batch loss.
Ideal UF/DF Scenario
Related Assets
Replacing offline process control with instant inline data offered by Process Analytical Technology ensures tight control over UF/DF. Monipa is successfully used to determine protein and various excipient concentrations with inline measurements during UF/DF in mAb downstream processing. Get in touch with us to explore tailored applications to your processes!

Case Study
Simultaneous Real-Time Monitoring of Protein and Sugars in UF/DF Process
Authors: AGC Biologics, IRUBIS

Application Note (2023)
Real-time infrared monitoring of an ultra-/diafiltration process
Authors: Adrianna Milewska, Clemens Minnich, Simon Kern
